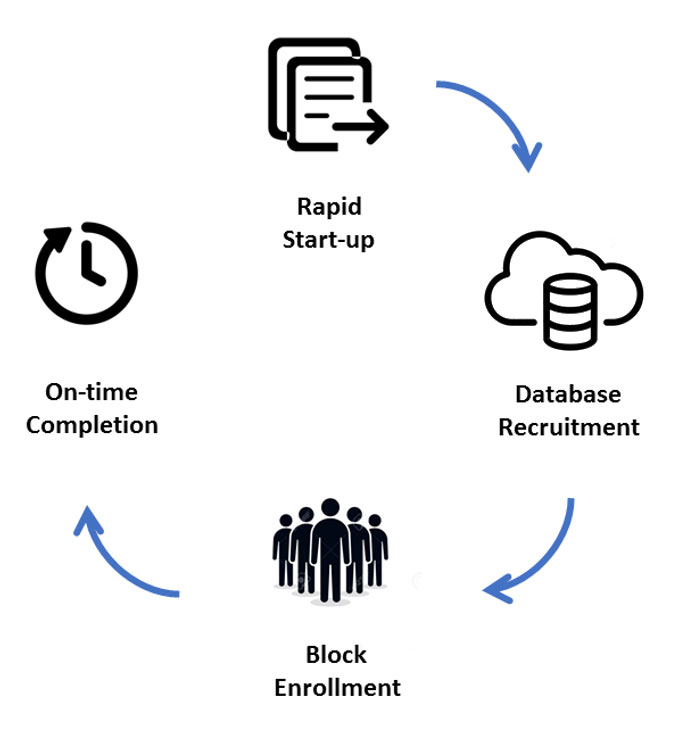
The KGL Advantage
We provide unmatched reliability in our capacity to activate, execute and complete studies within expected timelines.
Services offered throughout the full life cycle of clinical studies:
Design and Development
Study concepts, trial designs, protocol writing, source document creation, budgeting.
Clinical Trial Management
Electronic IRB submissions, randomization and blinding, product accountability, adverse event reporting.
Reliable Endpoints
Expert grading, imaging, image analysis, instrumentation, skin biopsies, subject questionnaire.
Expert Analysis
Biostatistics, summary conclusions, clinical study reporting.